We have several projects focusing on the use of machine learning in Radiation Oncology applications. Our focus is on identifying clinically-significant problems which can be solved using state of the art machine learning algorithms, in particular deep learning.
Current Projects
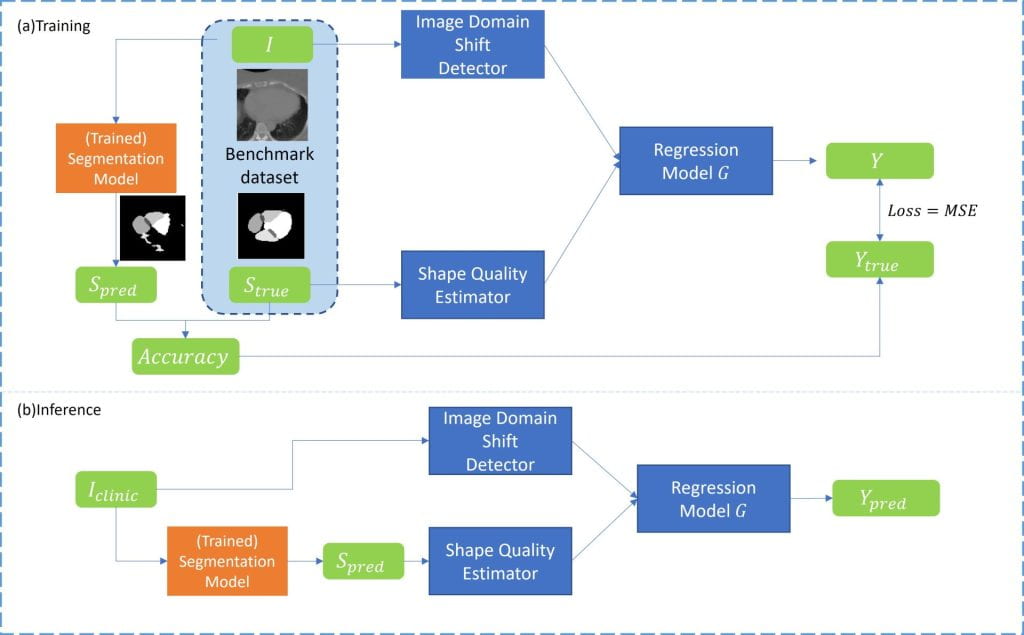
Quality assurance of AI systems
Because AI systems such as autosegmentation, synthetic CT generation, and the like are trained models that rely on historical data, there is a risk that model performance will degrade over time as new data varies from the historical data on which the model was trained. The goal of this project is to build monitoring approaches for clinical AI systems to ensure high quality and safe deployment and operation of clinical AI
Previous Projects
4DCT artifact correction
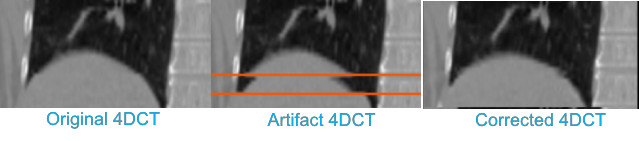
Motion-induced artifacts are present in more than 50% of clinical 4DCT scans. These residual artifacts stem from irregular breathing, selection of incorrect scan parameters (e.g., pitch too high), or changes in breathing rate during scanning. Even subtle artifacts can introduce error into delineation and IGRT processes, while gross artifact can require rescanning the patient, introducing treatment delay and additional unnecessary patient dose. We propose to develop and evaluate a retrospective method to mitigate artifact in already existing, reconstructed 4DCT images. The method requires only the reconstructed 4DCT images in DICOM format (no raw data or respiratory signal is required), which is advantageous compared to other potential solutions that require modification to the scanning system prospectively.
The following image shows a simple feasibility test of our method. Artifacts were introduced into the ‘ground truth’ image, which was a low artifact scan from the dir-lab dataset, through a simulation procedure. Our prototype method was then used to try to recover the original ground truth image. As can be seen qualitatively, the method appears to do well in this recovery.
Learning-assisted lung tumor target definition
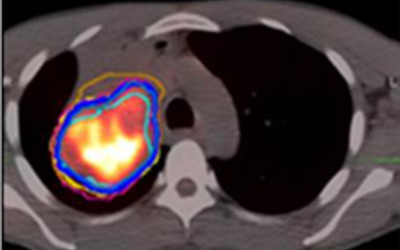
Radiographic changes following stereotactic body radiotherapy (SBRT) are associated with radiation‐induced lung injury (RILI=clinical radiation pneumonitis). Identification of predictive imaging biomarkers for RILI will allow individualized risk assessment and potentially enable prevention through individualized treatment planning or early intervention to reduce the risk of RILI in this vulnerable population. We will evaluate radiographic changes over time, including dose‐based assessment, and integrate these findings with clinical parameters to build an imaging biomarker for assessing individual RILI risk. Deformable image registration of repeated CT scans to correct for large radiation‐induced anatomical distortions of the lung and a radiomics approach with analysis of longitudinal variations of texture features will be employed to improve the power of the predictive model.
Understanding radiation-induced lung injury after SBRT
Target definition is one of the largest remaining geometric uncertainties in radiotherapy of locally-advanced lung cancer. One goal of this project is to use local texture to aid the physician in identifying the type of tumor to normal tissue interface (e.g., tumor/hilum, tumor/atelectasis), which could support assisted delineation or region-specific margins. In a second goal, image-based markers of early response of lung tumors to RT will be explored, using similar texture features. Both subprojects will explore texture features in multi-modality (MRI, CT, PET) imaging.
